LP Gas Fiasco - A Tragedy Rooted in A Failed Process Safety Management!
By Eng. (Dr.) Deshai Botheju
The ongoing explosions and gas leak accidents related to domestic LP gas cylinders have created an environment of fear, anger, and social unrest throughout the country. More than 400 explosions and gas leak incidents have been reported just during the first week of December 2021. In addition, a large number of observations have been made with respect to slowly leaking gas cylinder valves.
The reported accidents and incidents can be divided into four major categories: (a) Sudden gas explosions inside houses and building, (b) Exploding gas cookers, (c) Major gas leaks and resulting damages associated with the pressure regulator and the hoses, (d) Minor gas leaks from the cylinder valve, regulator, or the hoses. The number of accidents reported during a single week has far exceeded the typical gas related accidents happening within a typical year in the country. Something must have been gone terribly wrong for Sri Lankan LPG consumers ! Regrettably, several media reports now indicate multiple fatalities associated with these gas accidents. More people are likely to have received severe injuries as well. The associated property damage aspect comes in addition. Accordingly, this is now a “distributed major accident” scenario still ongoing in the country, with no or little control over it.
Characteristics of LPG
Liquified Petroleum Gas, abbreviated as LPG, is an energy carrier derived during crude oil refining or natural gas processing. These are called gas condensates according to petroleum industry terminology, and are often byproducts generated during the production of liquid fuels (like gasoline, diesel, and kerosene) or natural gas (i.e. methane). The key components of typical LP gas are propane (an alkane gas containing 3 carbon atoms – C3H8) and butane (an alkane gas containing 4 carbon atoms – C4H10). In addition, small amounts of propylene, methane, pentane and other minor constituents can be present. LP gases do not originally possess a clearly recognizable odor. Therefor, in order to identify any gas leaks, methyl mercaptan (CH3SH) or a similar odor generating component is added to LP gas before its commercial use. Table 1 provides a useful comparison between propane and butane, with respect to key physical /chemical properties. Many of these characteristics are very relevant for the discussion presented in this article.
Depending on the refinery process or depending on the intended use, the LP gas can have a widely varying composition difference between the propane and butane components. Under normal atmospheric pressure conditions, butane has the higher boiling point of -0.5 oC (minus 0.5 centigrade) compared to propane’s -42 oC (minus 42) boiling point. That means in colder climates where the ambient temperature could go below 0 oC, the LP gas must mostly contain propane in order to use that as a fuel gas (otherwise it wouldn’t flow as a gas, and butane would stay in the cylinder as a liquid). Therefore, the butane content is greatly reduced in LP gas used in colder climate countries (typically less than 5 % of the volume). For tropical countries like Sri Lanka, having a high butane content is just fine, as the year-round temperature is almost always above zero centigrade (except for some rare occasions in the hills). Further, butane is a much safer gas to use. This is due to its much lower vapor pressure (31 psi) compared to propane (124.5 psi). Therefore, the containment integrity requirements shall be much stricter for propane use compared to butane.
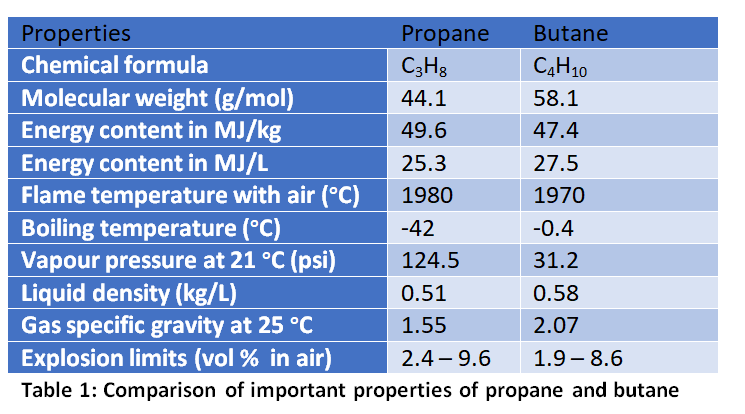
Composition changes and pressure effects
Unlike compressed gas cylinders, the LP gas cylinders are not filled with 100 % of gas. Instead, a newly filled cylinder would contain the liquids (hence the name LP gas) to about 85 % volume. Only the remaining 15 % ullage volume contains actual gas. These two phases (i.e. liquid and gas) are in equilibrium. The pressure within this gas filled ullage is the equilibrium pressure of the corresponding liquid mixture (of propane and butane). This equilibrium pressure can be predicted based on the ambient temperature and the composition of the liquid phase. Table 2 provides the values of these equilibrium pressures (in psig) for different propane-butane mixtures at the temperature of 32 oC (which is quite close to the typical ambient temperature condition in Sri Lanka).
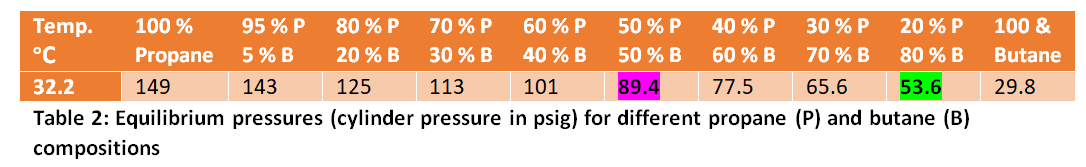
As can be seen from Table 2, at 32 oC temperature, a mixture of 80 % butane and 20 % propane has an equilibrium pressure of 53.6 psig. This was used to be the composition used in Sri Lanka for a long time. All appliances (including gas cookers), pressure regulators, hoses, hose connectors, and gas cylinder valves, and cylinders have been used to be accustomed to this pressure condition. In other words, our consumer gas utility system has been established at this pressure condition. Nevertheless, the gas cylinders themselves are manufactured to tolerate a much higher pressure.
If the butane -propane composition is suddenly changed to 50 % butane- and 50 % propane, now the increased propane content leads to a much higher equilibrium pressure of 89.4 psig. It is obvious that this is a very significant pressure increase from the previous condition that our systems have been used to tolerate.
Containment integrity issue
Increased propane content leads to a significant increase of the gas pressure inside the cylinder. This is because propane has a much higher equilibrium vapor pressure compared to butane (see Table 1). Now, the total customer side utility system can face a containment integrity problem. In other words, gas leaks are likely to happen from many of the system components. Table 3 elaborates potential impacts of this pressure increase on different system components. Figure 1 further illustrates the potential leak sources and pathways associated with the gas cylinder valve.

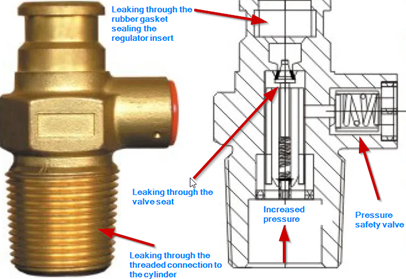 Figure 1: Potential leak sources associated with the LPG cylinder valve
Figure 1: Potential leak sources associated with the LPG cylinder valve
Quality of LPG accessories
The product quality of domestic gas system accessories available in Sri Lanka also plays a role in the ongoing crisis. For example, it is observed that even so called "good regulators" sold in SL are normally rated for about 7.5 bar maximum inlet pressure. Some of the “cheaper” regulators sold in the country have been found to be rated for just 6 bar or even less than that. Meanwhile, we have noted that typical “low pressure LPG regulators” manufactured or sold in Europe (e.g. Germany, Norway) are rated for a maximum inlet pressure of 16 bar. Now this says a whole lot about "Sri Lanka's problem" when it comes to domestic LPG accessories and their role in the current situation. Remember that when the LPG cylinder contains 50 % propane and 50 % butane, the headspace pressure will be close to 7.2 barg at 100 oF temperature (i.e. 37.8 oC ). Also note that many LPG consumers keep their gas cylinders close to the cooker; and as a result the surrounding air temperature may sometimes exceed 35 oC. This is especially the case with many roadside shops, restaurants, etc. due to space limitations issues. (For information: if a cylinder is filled with 100 % propane, the cylinder pressure will reach almost 12 barg at 100 oF temperature. On the other hand if a cylinder is filled with 100 % butane, then the cylinder pressure will only be less than 3 barg at 100 oF ). Interestingly, many pressure regulators sold in the country are specifically marked as “Butane”, thus justifying their low inlet pressure rating. Meanwhile the LPG regulators sold in many European countries are suitable for 100 % propane. Therefore, increasing the LPG composition towards higher propane concentrations without due attention to the current accessories and gas system equipment available in the country is a very imprudent and hazardous move, clearly indicating the level of immaturity of the process safety management practice in use. Note that, if the cylinder pressure exceeds the rated maximum inlet pressure of the regulator, then two things can happen: (i) higher likelihood of gas leaks from the regulator’s coupling point to the cylinder valve, (ii) higher pressure gas flow towards the gas cooker (exceeding the regulated outlet pressure - typically close to 30 mbar). If the increased gas flow exceeds the burner’s design limits, then the additional gas will escape without combustion and can create an explosive gas atmosphere near and around the gas cooker. Many of the reported “exploding gas cooker” accidents are likely to be due to this scenario.
What happens during a gas leak scenario ?
Propane and butane are flammable and explosive gases when mixed with air (or oxygen). Both of these gases can create an explosive gas mixture when mixed with air within the approx. volume percentages of 2 – 10 % (i.e. within LEL- Lower explosive limit and UEL – Upper explosive limit); see Table 1. Outside of this volume percentage range, the gas would not ignite. However, at higher gas concentrations, the gas cloud can still pose an asphyxiation hazard to humans as it replaces breathable oxygen in air
Even a very small gas leak happening through the cylinder valve, regulator, or from any other component (see Table 3 and Figure 1) can accumulate inside a building over several hours. Note that both propane and butane gases have a higher density compared to air (i.e. heavier than air; see specific gravity values shown in Table 1). That means when a gas leak occurs, the explosive gas cloud will basically accumulate close to the floor elevation (rather than moving upwards and dissipate). This situation is more likely to occur at night when the doors and windows are closed and hence no or very low ventilation possibility exists. Now if this leaking gas cloud reaches the LEL concentration limit within that surrounding (e.g. kitchen), then it is a bomb ready to be triggered at any time. Only it needs is a small spark, which may be created when an electrical switch makes contacts (on or off), or even due to static electricity present in the atmosphere (or due to an actual flame such as lighting a match). At that moment, an explosive combustion reaction occurs within the flammable gas cloud and the releasing energy content is transmitted as a pressure wave accompanied often by a fire ball. This is a typical atmospheric gas cloud explosion. Secondary damages can occur due to projectile effects (e.g. broken glass), prolonged fires, collapsing roofs and walls, etc.
Change management failure
Changing an existing LP gas composition without a detailed safety assessment is an act of sheer negligence almost reaching the level of absurdity. It’s a fundamental process engineering principal to follow a comprehensive Management of Change (MoC) protocol before doing this kind of (or even far less consequential than this) change to a product, process, or an operating procedure. Even a Process Engineering intern (trainee) can explain this to a production management. As part of an MoC process, it is absolutely necessary to conduct a dedicated risk assessment or a standard safety study such as “HAZards and Operability” study (HAZOP) or HAZards IDentification study (HAZID). If such HAZID or HAZOP study had been conducted in this case, all of the problems indicated in Table 3 (including the issues related to accessories), plus more, could have been identified in advance, thus avoiding the calamity unfolded, thereby saving valuable human lives and properties.
The cost factor and energy contents
The heat energy contents of propane and butane are respectively 49.58 and 47.39 MJ/kg (megajoule per kilogram). However, the density of liquid propane and butane are 0.51 and 0.58 kg/L respectively. That means due to the lower density of propane compared to butane, propane has a slightly lower energy content when based on volume (25.3 and 27.5 MJ/L respectively). Nevertheless, propane burns with a slightly higher flame temperature compared to butane (1980 vs 1970 oC in air). In certain gas burners, propane could burn with slightly higher efficiency compared to butane (i.e. less deposition of carbon).
If calculated based on the heat energy content delivered (measured in BTU-British Thermal Units), propane is often a cheaper energy commodity compared to butane in the world energy market. Therefore, an LP gas mixture rich in propane can be cheaper. Also LP gases with more propane are easier to procure. While per BTU price is being cheaper, if calculated based on metric ton price, one might mislead others by saying that propane is more expensive than butane. That becomes a false statement if all gas pricing and market economics are based on the value of BTUs delivered to the customer (i.e. the customer is made to pay for the heat energy content delivered to them, and not for the weight of the gas). Also note that the exact price of a certain LPG shipment can be very different from the typical spot prices prevailing in the world energy market.
A safety culture issue
Every organization has a certain culture of safety. Without going into details of academic definitions of safety culture concept, we can still try to understand different characteristics of good (positive) safety cultures compared to bad (negative) safety cultures.
In a good safety culture: Management of Change (MoC) protocols are always followed; When an accident or an incident happens, it will always be investigated to the fullest extent and all learning lessons will be extracted; Always maintain transparency and honesty; Accept their own faults instead of finger-pointing towards others; No attempts of hiding information and baseless denials; Safety is always given priority over marginal economic gains. In contrast, the complete opposite of these would happen in an organization possessing a negative safety culture. Within such negative safety culture, far more dangerous accidents can happen.
Investigation and compensation
Any investigation into the recent series of unfortunate gas accidents happened in Sri Lanka must not just stop at identifying plausible physical causes. Such investigation must definitely look deeper into the related organizational factors, and must make necessary recommendations to bring about much needed organizational reforms in the form of enhancing safety culture. In addition, more systematic safety management requirements and stricter regulatory reforms must be recommended to avoid repetition of this kind of “organizationally rooted accidents”. Failing to do so may lead to greater disasters of higher magnitude, claiming multiple human lives in future.
Prompt compensation to those who faced harm must be a priority. Even more urgent is to recall every single gas cylinder delivered with hazardous pressure conditions, irrespective of the fact that the gas has been used or not. Like explained before, LP gas cylinders will retain the same high pressure condition until the last drop of liquid phase is vaporized. Therefore, totally unused as well as almost fully used gas cylinders will pose the same level of risk !
Note: The facts presented in this article are based on information available in the public domain. The analyses and opinions are based on the author’s long-term experience in the industry, and are not connected to any institutional interests.
 Eng. (Dr.) Deshai Botheju
Eng. (Dr.) Deshai Botheju
[Ph.D. (NTNU), M.Sc.Tech. (USN Norway), M.Sc. & B.Sc.Eng.(1st cl. Hons) (UoM), AIChE, AMIE(SL)]
The author is currently consulting for the Norwegian petroleum industry, within his field of expertise of Safety and Sustainability Engineering & Management. The key areas of his industrial experience and research interests include; Process safety & safety barriers design, Sustainability & Industrial safety risks management, and Safety culture.




