Current Status and Outlook for the Industrial Pipeline Corrosion
By Eng. (Dr.) G.I.P De Silva and Mr. M.B.A. Deemantha
Introduction
Corrosion can simply be known as a natural process that converts a refined metal into a more chemically stable form. This natural process becomes a concern when the machineries, equipment, tools and pipelines start to get corroded in the industry. Further, poor inspection of corrosion may end up with catastrophic failures of the various structures and components. Therefore corrosion in the industry has a potential to cause widespread damages across multiple economic sectors. The costs attributed to corrosion damages occurred in the industry have been estimated to be 3% to 5% of gross national product of industrialized countries. Therefore, corrosion has spawned an entire sphere of scientific study and engineering research.
Out of all industries, oil and gas industry plays a significant role when it comes to the corrosion damages because crude oil and natural gas carry various high impurity products which are inherently corrosive. Corrosion costs US industries alone an estimated $170 billion a year in which the oil and gas industry takes more than half of these costs. Carbon dioxide (CO2), hydrogen sulfide (H2S) and free water are the highly corrosive media that frequently get touched with oil and gas components. Generally, the components such as pipelines, valves and turbines are subjected various types of corrosion such as sweet corrosion, sour corrosion, oxygen corrosion, galvanic corrosion, crevice corrosion, erosion corrosion, microbiologically induced corrosion and stress corrosion cracking. The sour corrosion is a very specific corrosion type identified in the petroleum industry since H2S emission at refinery process is considerably high. The article mainly focuses on the sour corrosion in the industry specially its behavior, impacts to the industry and prediction models. In addition, other corrosion types found in the industry are also taken into considerations.
Sour corrosion can mainly be found in petroleum pipelines. The corrosion occurs when H2S is in wet condition. Formation of FeS (Mackinawite) through dissolution of metal is the core mechanism of sour corrosion. The concentration of H2S is a major factor of the behavior of sour corrosion. In addition to the H2S concentration (pH value), it has been found that the applied stress/pressure, time duration, iron sulfide scale, temperature, flow effects, solution composition and other chemicals such as inhibitors, glycol, biocides, demulsifies etc. effect the behavior of sour corrosion.
Since petroleum refineries are rich with aforementioned factors, this corrosion has become a critical problem that requires immediate and permanent solutions in petroleum industry. The best way to avoid sour corrosion is removing the factors that affect sulfide stress corrosion. But it is impossible due to the sour environment which presence in the petroleum industry. During the past fifteen years, the petroleum industry focused on the production of natural gas from deep wells (Sour wells). It is obvious that the pressure inside these deep wells gets a comparatively higher value. Sometimes, the pressure created information of natural gases exceeds 140 MPa. This high pressure inside sour wells makes likelihood for the contamination of hydrogen sulfide with natural gases.
Instead of preventing sour corrosion, identifying it at early stage is a practical approach to avoid the adverse effects of the corrosion. Some major failures and accidents have been occurred due to sour corrosion in the petroleum industry since it propagates without giving a prior warning. The existing techniques which are used to track sour corrosion do not considerably predict the behavior of corrosion. They indicate the extent that the corrosion has propagated. The existing techniques such as non-destructive testing, scanning electron microscope image analysis, optical techniques and electro chemical techniques cannot be used to predict the propagation of sour corrosion. These techniques are used in time basis inspection routines and these inspection routines do not reveal corrosion propagation rate or any other information that helps to estimate the time duration that the failure may occur.
Prediction models have been developed over the past fifteen years to predict the sulfide stress corrosion but most of them have failed to predict the corrosion rate or corrosion behavior accurately. The rest of the models have predicted the accurate results under certain conditions. Some of the models tend to predict wrong results when they are predicting corrosion behavior at higher time durations. Some of the models predict accurate results when they are predicting corrosion behavior at very low H2S concentrations while some models predict accurate results at high H2S Concentrations.
Petroleum refineries in Sri Lanka are also suffering from various types of corrosion including crevice corrosion, galvanic corrosion, pitting corrosion and sour corrosion etc. The main issue associated with the sour corrosion is formation of FeS layers inside the hot stream product line which carry H2S rich gas around pressure of 45 bars at around 380 0C. Generally the H2S content of the gas which is transmitted through these pipelines is 5% – 6 %. The non-destructive techniques such as magnetic particle tests, magnetic flux leakage testing, flow detection techniques and radiography testing etc are used for the inspection of sour corrosion. The pipelines are replaced only if the detected corrosion damage is serious. Otherwise O2 flows are used to burn corrosion products.
Although corrosion becomes one of main concerns of most of the countries, Sri Lanka is not paying a satisfactory attention for corrosion related research projects and innovations. The reason might be the few industries which are directly influenced by the severe corrosion effects, established within the country. On the other hand, Sri Lanka has made various claims about oil in the Mannar basin and elsewhere. Therefore, the country should have a proper disposition for the petroleum industry.
Major corrosion related accidents and failures
Corrosion is a phenomenon that effects severely in different industries. Bhopal accident is the biggest industrial failure in the history. The accident happened at a pesticide plant in Madhya Pradesh, India in 1984. The official death toll is 2259. But the unofficial news sources say that the real death toll goes beyond 8000.The root cause for the failure was identified as the poor maintenance of safety valves. Penetration of large amount of water into the tank filled with methyl isocyanate has caused for the chemical reactions which form toxic gases. The increase of pressure inside the tank had caused for an explosion blowing cloud of toxic gases. The company identified later that it was failed to identify the increase of pressure inside the tank since the pressure build up was extremely rapid. The failure analysis has found that the reason for this sudden pressure build up is the high speed of chemical reactions which was accelerated by corrosion products inside the tank.
 Figure 1: Toxic gas spreading in the city Bhopal.
Figure 1: Toxic gas spreading in the city Bhopal.
 Figure 2: A death body of a child who live near the factory.
Figure 2: A death body of a child who live near the factory.
Another large scale failure is the explosion of a gas pipeline operated by the El Paso Natural Gas Company (EPNG) adjacent to the Pecos River near Carlsbad, New Mexico. The explosion was happened due to the leakage of natural gas across the pipeline which was later found that it had been internally corroded.
 Figure 3: The crater that was created in the explosion of the gas pipeline.
Figure 3: The crater that was created in the explosion of the gas pipeline.
.
The pipeline corrosion is abundant in oil and gas related industries because they provide ideal environments for corrosion. Scientific and policy reports published by joint research center at European commission states that more than 80% of accidents happen in petroleum industry linked with corrosion phenomena. The same report stresses that these accidents can even be categorized based on the substances which causes for the failure. Table 1 shows process related substances cited as contributing to corrosion failures.
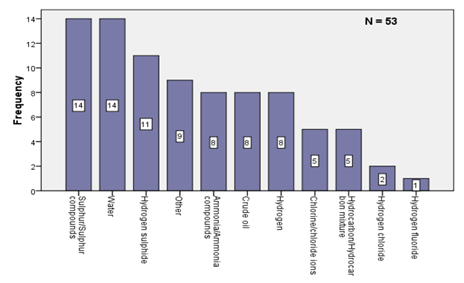 Figure 4: Process related substances cited as contributing to corrosion failures.
Figure 4: Process related substances cited as contributing to corrosion failures.
Table 1 illustrated that most of the corrosion failures have happened due to Sulphur/ Sulphur related compounds and hydrogen sulfide. The reason for this illustration could be found as the refinery processes emit higher amounts of hydrogen sulfide gas (H2S) to the factory environment.
Hydrogen sulfide corrosion and sulfide stress corrosion can be identified as highly prevailed types of sour corrosion in the industry. The hydrogen sulfide corrosion is simply the formation of FeS through direct heterogeneous reactions happens between Fe2+ ions and S2- or HS- ions. The sulfide stress corrosion is one step forward compared to the hydrogen sulfide stress corrosion since there is a crack formation due to the effect of H2S gas. The other main difference of sulfide stress corrosion is that there should be a tensile stress, which may be well below to metals yield stress value in the sour environment. Both of the corrosion types have been identified as significant phenomenon in the industry since the mechanisms of corrosions are little mysterious.
Corrosion detection techniques used in petroleum industry.
Mainly, there can be identified three corrosion detection techniques in the petroleum industry which are excavation detection technology, internal detection technology and external detection technology. In the excavation detection technology, the anti-corrosion coating defects of petroleum pipelines are detected first, then the detected location is excavated for the identification of the corrosion effect. The popular techniques which are used in the industry under this technology are pipeline current method (PCM), DC voltage gradient method (DCVG), and Pearson method.
 Figure 4: An accidental excavation detection of a pipeline.
Figure 4: An accidental excavation detection of a pipeline.
When it comes to the internal detection technology, the corrosion cracks are identified through the direct calculation of wall thickness. The popular techniques come under this technology are ultrasonic test method (UT), Magnetic flux leakage inspection (MFL) and eddy current testing.
 Figure 5: A pipeline eddy current testing apparatus under inspection
Figure 5: A pipeline eddy current testing apparatus under inspection
The external detection technology has the ability to detect the wall thickness of buried metal pipeline on ground surface. Common techniques used under this technology are transient electromagnetic method (TEM), metal magnetic memory testing method (MMM) and ultrasonic guided waves method (UGW).
 Figure 6: Detection of pipeline corrosion through transient electromagnetic method.
Figure 6: Detection of pipeline corrosion through transient electromagnetic method.
Each detection techniques are carried out in the industry as scheduled or unscheduled inspection routines. Although each technique has its own advantages and disadvantages, one common and serious disadvantage can be found, that is, when the inspections are carried out and corrosion is identified, the corrosion should have already occurred. Therefore if the corrosion behavior is random or unpredictable, these techniques cannot be used to identify corrosion before the failure of component occur. As a remedy for this issue, engineers and researchers have suggested the concept of the development of prediction models.
Development of a proper corrosion prediction model
The most challenging problem of sour corrosion is the unpredictable behavior of the initiation and propagation of corrosion. Because of this reason, development of a prediction model at all conditions has become a difficult task. Research groups of S. Nesic, Wei Sun, Stephen N Smith and Yougui Sheng at Ohio University, Y.P. Asmara at University Malaysia Pahang have given a significant contribution for development of the sour corrosion prediction models. A research conducted by the authorsof this article revealed that the corrosion initiation has a unique time duration under a defined environment. Further they revealed that the behavior of the propagation of corrosion highly depend on the time duration in addition to the sourness of the environment and the tensile stress of the metal. The propagating direction of the initiated corrosion vary when the time duration get increased. Therefore it can be stressed that a single model cannot be used to predict the propagation of sour corrosion. A set of models which was developed for set of conditions should be combined to develop a proper prediction model which has the ability to predict the corrosion at all conditions.
 Eng. (Dr.) G.I.P De Silva
Eng. (Dr.) G.I.P De Silva
Dr. G.I.P. De Silva is a senior lecturer in the Department of Materials Science and Engineering, University of Moratuwa and also a Charted Engineer. He has obtained a M.Phil research degree on “Enhancement of Surface Quality of Brass Castings Cost Effectively Using Locally Available Sand and Clay Types” from University of Moratuwa and completed his Ph.D degree on “Oxide Superconductor Thin Films’’ at Kochi University of Technology (KUT), Japan. His research and teaching areas mostly focused on industrial metallurgy and characterization of oxide superconductor thin films. Dr. G.I.P. De Silva has published two books, many indexed journal papers and obtained two patents on foundry technology.
 Eng. M.B.A. Deemantha
Eng. M.B.A. Deemantha
Eng. (Mr) Akesh Manimendra is a research assistant in the Department of Materials Science and Engineering, University of Moratuwa. He has completed his master’s degree on “the development of a model to predict the propagation of sulfide stress corrosion of petroleum pipelines” at University of Moratuwa. He has served as a residential and a visiting lecturer in British institute of Engineering and technology – Sri Lanka. His research and teaching areas mostly focused on corrosion and degradation of materials. Mr Akesh Manimendra has published two research papers on the propagation of sulfide stress corrosion of petroleum pipelines.




